Bispecific Antibody (BsAb) Design, Expression and Purification
Bispecific antibody (BsAb) is one kind of the next generation of antibody. The dual targeting is a particular concept linked with bsAbs, enabling them to target two different antigens on two different cells, two different antigens on the same cell, or two different epitopes on the same antigen.
According to the existence of the Fc (fragment crystallizable) region, bispecific antibodies can be divided into two categories: IgG-Based bsAbs and Fragment-Based bsAbs.
1. IgG-based bispecific antibody (BsAb)
IgG-Based bispecific antibodies are similar in structure to native antibodies, and all have Fc regions.
To prepare bsAbs in IgG format, two challenges should be met. One is heavy chain heterodimerization, in which that each heavy chain will only pair with the heavy chain of the second specificity. The second is cognate heavy chain and light chain paring, in which each heavy chain will only pair with the light chain of its own specificity.
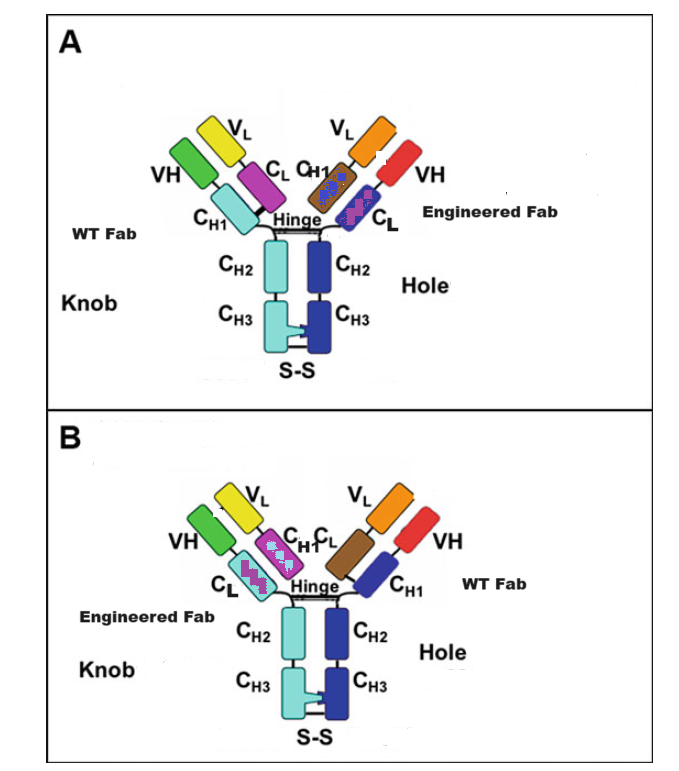
1.1 IgG-like Bispecific Antibody CrossMAbCH1-CL
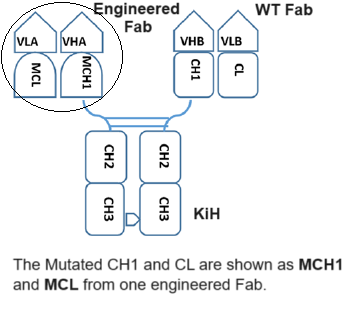
The CrossMAb technology is based on the crossover of the antibody domain within ONE Fab-arm of a bispecific IgG antibody to enable correct cognate heavy chain and light chain paring. The favorite format is CrossMAbCH1-CL, an engineered Fab carrying a CH1-CL domain crossover, without side products are formed. Whereas, the correct heavy chain heterodimerization can be enforced by mutation through the knobs into holes (KiH). This platform has proven to be one of the most versatile antibody engineering technologies, allowing the generation of various bispecific antibody formats, including bi-(1+1), tri- (2+1) and tetra-(2+2) valent bispecific antibodies, as well as non-Fc tandem antigen-binding fragment (Fab)-based antibodies. These asymmetric CrossMAbs can be produced using the well-established IgG production workstream based on one single standard Chinese hamster ovary cell line and typical upstream and downstream processing. The product is comparable in scale, yield, glycosylation, stability and quality to conventional IgG antibodies.
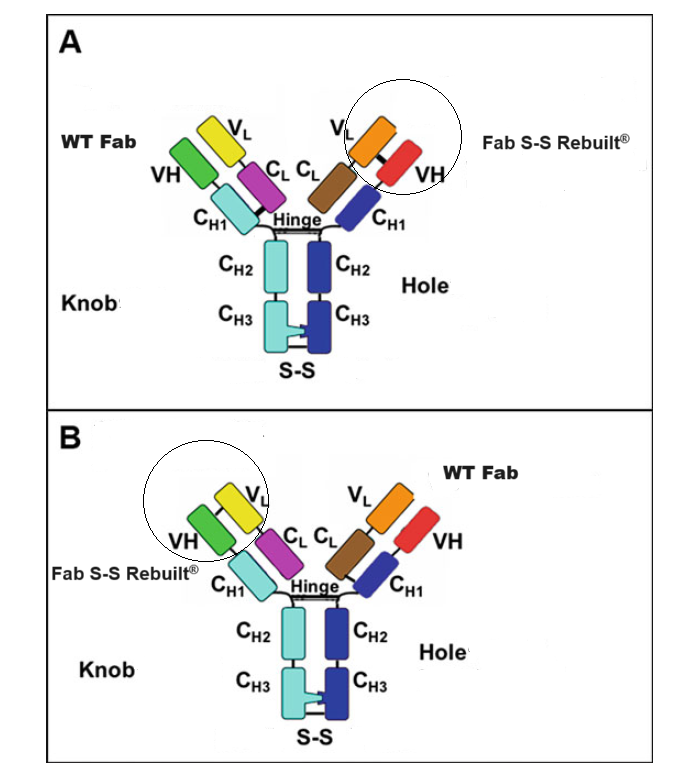
1.2 IgG-like bispecific antibody Fab S-S Rebuilt®
Oak Biosciences develop an optimized platform to meeting the correct both heavy chain heterodimerization through KiH (knobs into holes) and correct cognate heavy chain and light chain paring through our own Fab S-S Rebuilt technologies, in which an engineered disulfide bond between the antibodies’ variable domains that asymmetrically replaces the natural disulfide bond between CH1 and CL.
1.3 IgG4-like Bispecific Antibody (BsAb)
IgG4-like BsAb is a full length IgG-based BsAb. It is designed through KiH (heavy chain heterodimerization) and CH1-CL cognate heavy chain and light chain paring and interface through mutation replaced by T-cell receptor α/β constant domain to get bivalent IgG4 BsAb.
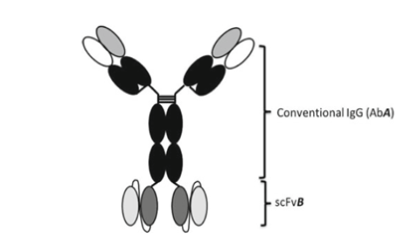
1.4 IgG-like Tetravalent IgG-scFv BsAb
Both heavy chain heterodimerization and CH1-CL cognate heavy chain and light chain paring are meeting through DNA recombinant and cloning platform. IgG–scFv fusion, in which a scFv of one antigen specificity is fused to the c-terminal of a conventional IgG of a different antigen specificity. In construction of the IgG-scFv BsAb molecule, Fab A is first reformatted to a conventional IgG (Ab A). The VH and VL domains of Fab B are cloned and assembled into an scFv (scFv B) in the orientation, VH-(GGGGS)3-VL, and scFv B is then fused to the c-terminal of Ab A IgG CH3 domain. The IgG-scFv produced is purified by one- step Protein A affinity chromatography and retained high binding affinity to both target A and target B.
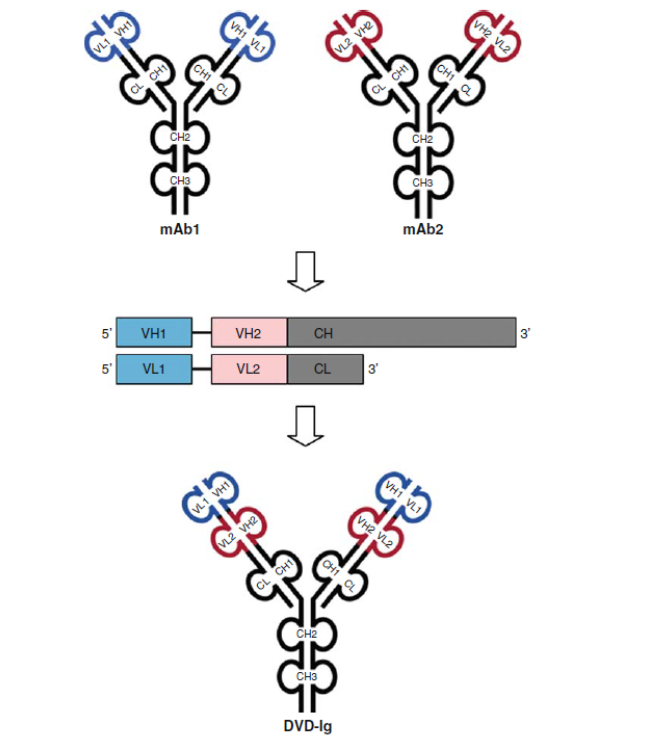
1.5 IgG-like Dual-variable domain Ig (DVD-Ig) BsAb
Both heavy chain heterodimerization and CH1-CL cognate heavy chain and light chain paring are meeting through DNA recombinant and cloning platform. The DVD-Ig is designed as an IgG-like molecule, except that each light chain and heavy chain contains two variable domains in tandem through a short peptide linkage, instead of one variable domain. DVD-Igs are highly stable in vivo and exhibit excellent IgG-like physicochemical and pharmacokinetic properties.
2. Antibody fragment-based bispecific antibody (BsAb)
Fragment-based bsAbs are composed of the variable light and heavy domains from two antibodies, or the Fab units, and lack the Fc region which distinguishes them from IgG-Based bsAbs.
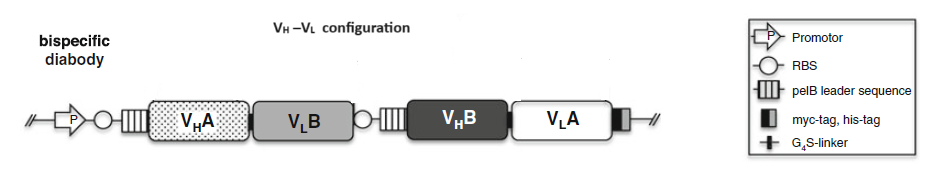
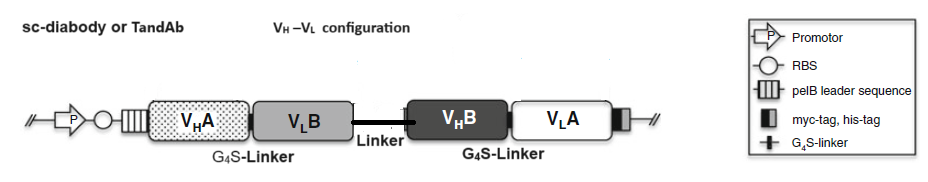
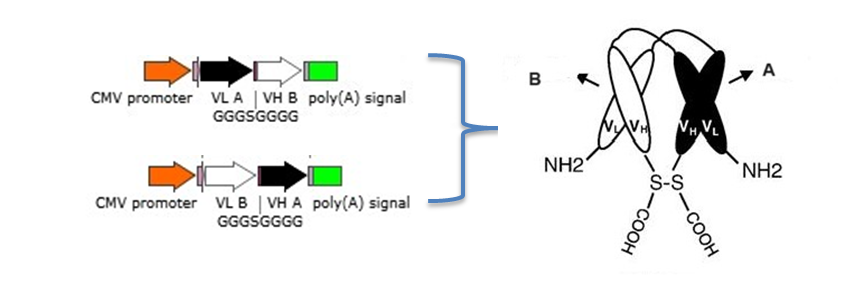
2.1 Bispecific diabodies, dimeric Single chain Diabodies (sc-diabodies) and tetrameric tandem diabodies (TandAb)
The procedure to design and make different disbodies are
1) Construction of genes encoding hybrid scFvs consisting of the VH domain from one Ab A (VH A ) connected by a 5–10 amino acid linker to the V L domain of another Ab B (VL B ) and the VH domain from one Ab B (VH B ) connected by a 5–10 amino acid linker to the V L domain of another Ab A(VLA );
2) Construction of a dicistronic operon for coexpression and co-secretion of two hybrid scFvs, VH A – VL B and VH B – VLA , with the formation of functional heterodimer (diabody) in the bacterial periplasm to make bispecific Diabodies;
3) Joining the two dimerizing parts, VH A – VL B and VH B – VLA, with a peptide linker of 10–20 amino acids in length to make single chain-diabodies (scDb) or tandem diabodies (TandAb).
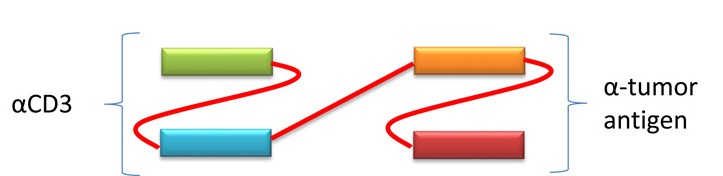
2.2 Bispecific T cell enagger (BiTE)
A recombinant antibody comprised of two tandem scFv, one binding CD3 on T cells, the other targeting a tumor antigen on tumor cells. BiTE constructs have the unique ability to engage any type of T-cell since the interaction does not involve co-stimulation or necessitate the involvement of a major histocompatibility complex.
2.3 Bispecific dual-affinity Re-targeting molecules (DARTs)
DARTs are bispecific antibodies designed to simultaneously recognize both tumor antigens and invariant epitopes of the CD3/TCR complex on T cells. By doing so, they effectively recruit T lymphocytes to tumor targets in a noncognate fashion, bypassing the MHC context required for antigen recognition by the TCR. Theoretically, this enables all T cells to be redirected against the tumor. The DART (bispecific against B-cell C19 and T-cell CD3 structure) facilitates T-cell/B-cell associations, leading to potent redirected T cell–killing of B-cell lymphoma. DARTs generally are expressed in mammalian cell system CHO or HEK293.
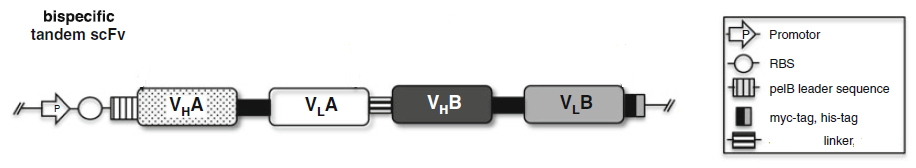
2.4 Bispecific tandem scFv: scFvA:scFvB
To making scFv based BsAb, scFv A and scFv B are separated by around a 25 amino acid flexible linker.
Our services Provides two expression platforms:
1) E. coli bacterial expression system, which is seamlessly compatible with our phage display library construction, antibody phage panning and candidate validation projects.
2) Mammalian transient expression systems in CHO or HEK293 cells.
What We Offer:
1. Antibody Fragment based BsAbs or IgG-based BsAbs design and produced from Synthetic DNA– This is the fastest route to construct BsAbs. Oligonucleotides are first assembled in vitro and then cloned into our in-house expression vectors. The BsAb is then expressed in E. coli or a mammalian cell line. The turnaround time is 1-2 weeks.
2. Antibody Fragment based BsAbs or IgG-based BsAbs design and produced from monoclonal cell line – VH and VL are obtained through the customer provided monoclonal hybridoma cells. The mRNA from the monoclonal will be cloned to create a cDNA vector from which the variable heavy (VH) and light (VL) chains are then subcloned into expression vector(s).
3. Antibody Fragment based BsAbs or IgG-based BsAbs design and produced from accession numbers – The VH and VL of the target monoclonal antibodies are synthesized according to the accession number, cloned, expressed, purified.
Key Features
- Codon optimization technology
- Seamless cloning technology
- Two expression systems (either bacterial induction expression system or mammalia transient expression system)
- Evaluation available, and scale-up option
Process Overview
Client provides: Antibody sequence, plasmids containing coding sequence, or hybridoma cells (>1×10<sup>5</sup> cells, in liquid nitrogen or dry ice) with isotype or class information
Stage I: Variable Region (VH, VL) Sequencing or Gene Synthesis
Turnaround time: 5 days
Stage II: Bacterial expression vector or mammalian expression vector construction and sequence validation
Turnaround time: 5 days
Stage III: Transient expression or bacterial expression and affinity purification
Turnaround time: 1-2 weeks
Deliverables:
1) Purified BsAbs
2) Certificate of Analysis (COA) data sheet
Ordering Information
Antibody Fragment-based or IgG-based bispecific antibody design and production Services
| Catalog | Description | Unit | Price |
|---|---|---|---|
| 82111 | Antibody fragment-based diabodies BsAds design and expression and affinity purification | 1 Project | Contact Us |
| 82107 | Antibody fragment-based DARTs BsAds design and expression and affinity purification | 1 Project | Contact Us |
| 82113 | Antibody fragment-based tandem scFv BsAbs design and expression and affinity purification | 1 Project | Contact Us |
| 82108 | Antibody fragment-based BiTE BsAbs design and expression and affinity purification | 1 Project | Contact Us |
| 82115 | IgG-based BsAbs design and expression and affinity purification | 1 Project | Contact Us |